Lab Introduction
Members' Pages
Principal Investigator
Research Professor
Ph.D. Students
M.S. Students
Undergraduate Students
- Eunseo Hong
- Ramyun Lee
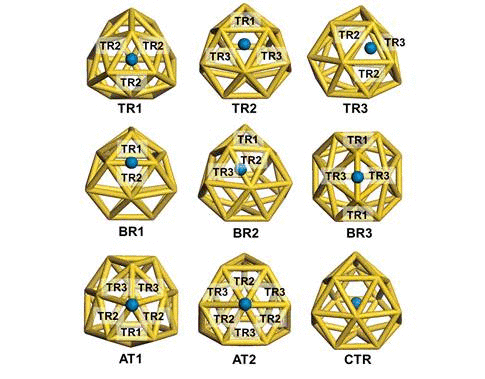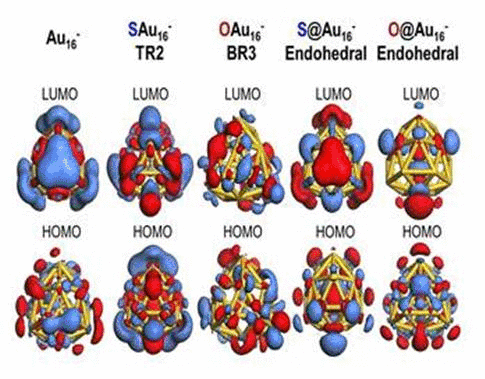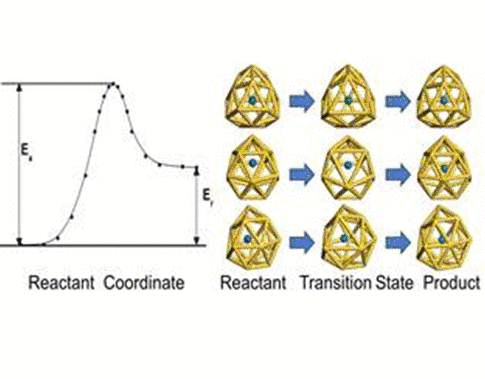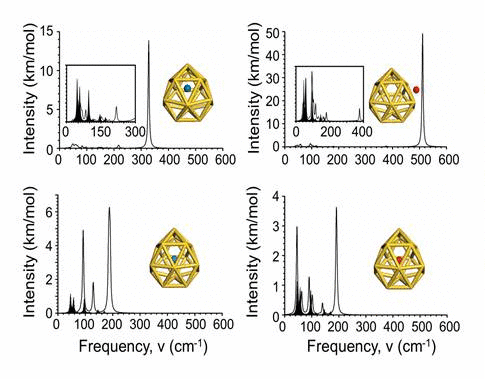
We have investigated the adsorption properties of chalcogen elements
(oxygen and sulfur) and nitrogen on an anionic golden fullerene
Au16- and its effects on the structural and
electronic properties of the golden cage. In particular, we found that
when a sulfur atom is encapsulated inside Au16-,
its bonding character with Au atoms appears ionic due to electron
transfer from sulfur to the gold nanocage. In contrast, the
exohedrally adsorbed S atom tends to have strong orbital hybridization
with the gold nanocage. For an oxygen adsorption case, electrons from
the golden cage tend to be shared with the adsorbed O atom exhibiting
strong orbital hybridization, regardless of its adsorption sites. To
investigate the transition behaviors between the most stable exohedral
and endohedral adsorption configurations, we calculate the activation
and reaction energies in the transition. The oxygen atom experiences a
lower energy barrier than the sulfur atom due to its smaller atomic
radius. Finally, we explore the vibrational properties of S- or
O-adsorbed Au16- 16 buckyballs by calculating
their infrared spectra. When the N atom is adsorbed on the cage,
electrons are transferred to nitrogen from Au 16. The nitrogen atom
may move thermally from the exterior to the interior through a bridge
site. In infrared spectra, exohedral doping causes greater intensities
at higher frequencies than endohedral doping.
 download:
download: 

 download:
download: 
