Lab Introduction
Members' Pages
Principal Investigator
Research Professor
Ph.D. Students
M.S. Students
Undergraduate Students
- Eunseo Hong
- Ramyun Lee
We have investigated various properties of divers gold nanostructures using ab initio density functional theory with spin-orbit coupling.
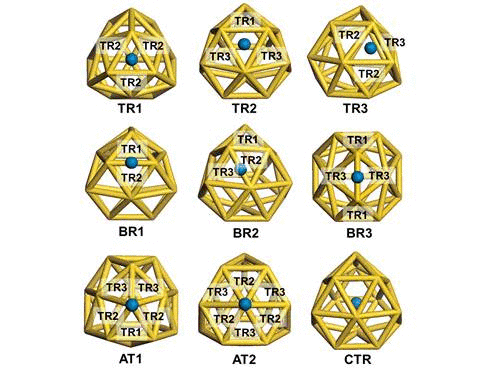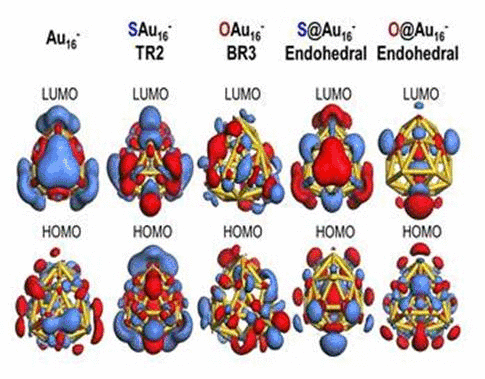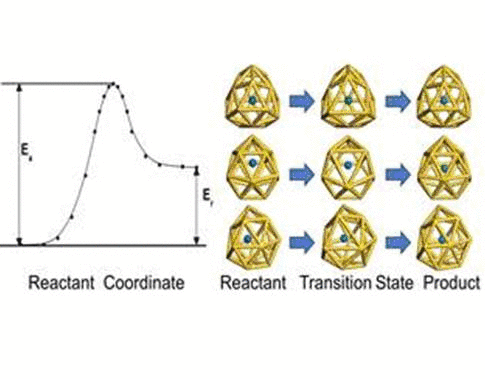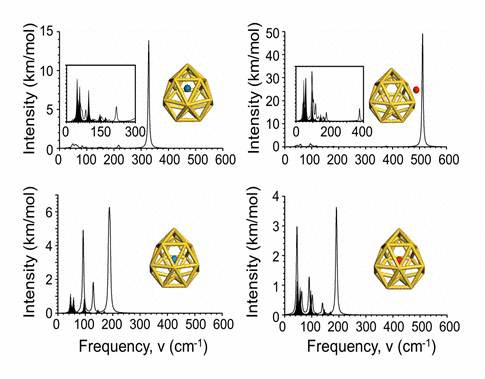
We have investigated the adsorption properties of chalcogen elements
(oxygen and sulfur) and nitrogen on an anionic golden fullerene
Au16- and its effects on the structural and
electronic properties of the golden cage. In particular, we found that
when a sulfur atom is encapsulated inside Au16-,
its bonding character with Au atoms appears ionic due to electron
transfer from sulfur to the gold nanocage. In contrast, the
exohedrally adsorbed S atom tends to have strong orbital hybridization
with the gold nanocage. For an oxygen adsorption case, electrons from
the golden cage tend to be shared with the adsorbed O atom exhibiting
strong orbital hybridization, regardless of its adsorption sites. To
investigate the transition behaviors between the most stable exohedral
and endohedral adsorption configurations, we calculate the activation
and reaction energies in the transition. The oxygen atom experiences a
lower energy barrier than the sulfur atom due to its smaller atomic
radius. Finally, we explore the vibrational properties of S- or
O-adsorbed Au16- 16 buckyballs by calculating
their infrared spectra. When the N atom is adsorbed on the cage,
electrons are transferred to nitrogen from Au 16. The nitrogen atom
may move thermally from the exterior to the interior through a bridge
site. In infrared spectra, exohedral doping causes greater intensities
at higher frequencies than endohedral doping.
 download:
download: 

 download:
download: 

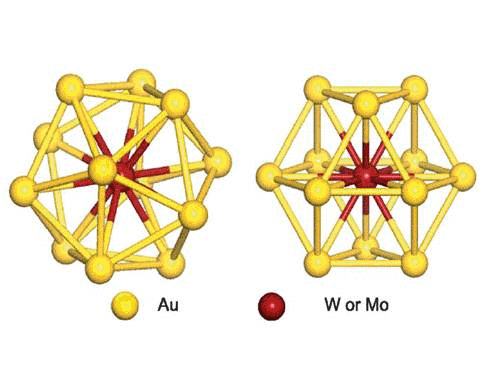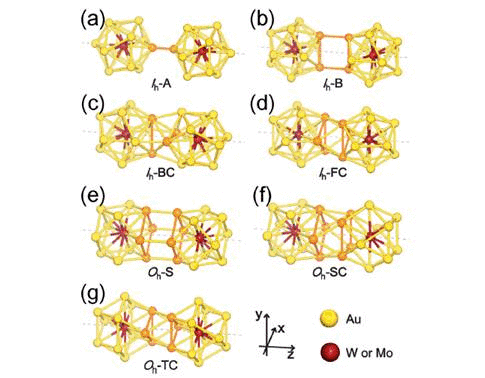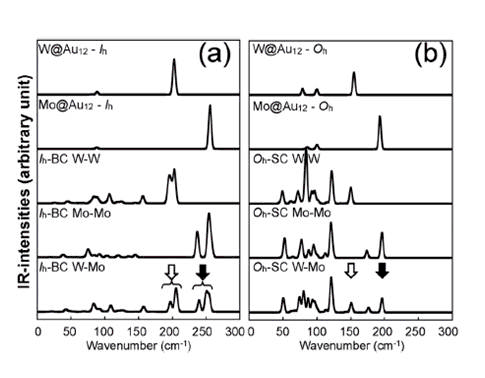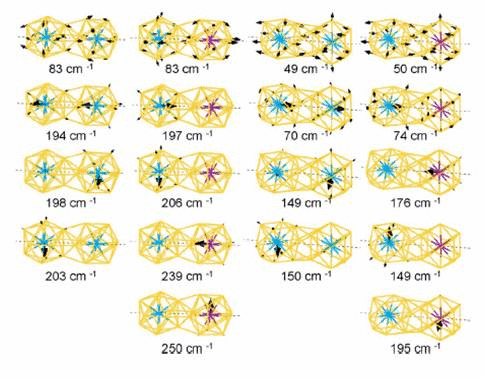
We have studied the dimerization of metal-encapsulated gold
nanoclusters M@Au12 (M = W, Mo) with Ih or
Oh symmetry, and their structural and electronic
properties.To determine the most stable dimer structure in each case,
various configurations are considered. We find that during dimerization,
gold atoms near the interface tend to form inter-cluster triangular bonds,
which stabilize two monomer clusters by about 3.3–3.5 eV.
The dimerization along a specific axis selected as the z axis causes
the symmetry reduction of each M@Au12 cluster resulting in the modification
of electronic structures. It is found that all the stable dimers exhibit
a much smaller HOMO–LUMO gap than those of their comprising monomers.
Such a gap decrease is mainly attributed to the dz2 orbital splitting of the
central atoms owing to dimerization. We also calculate the vibrational modes
and the corresponding IRactive spectra, which are distinguishable
for different dimer configurations. In addition, we find that the
IR-active modes of the Oh-based dimer structures appear to be red-shifted
in comparison to those of Ih-based ones.
Thus, the IR spectra may be utilized experimentally to discriminate dimer
configurations with different central metal atoms and/or dissimilar structural symmetries.
 download:
download: 

We have studied the 1D chain formation of metal-encapsulated gold nanoclusters M@Au12 (M = W, Mo) with Ih or Oh symmetry, and their structural and electronic properties.